Computational and Hardware Adaptive-Optics OCT
Standard OCT resolution is limited compared to traditional microscopy techniques making it challenging to resolve fine structures such as cells and fibers. Therefore, several efforts have been made to achieve high-resolution OCT imaging including post-processing methods and specialized hardware configurations. Among post-processing methods, computational adaptive-optics together with either computational refocusing or interferometric synthetic aperture microscopy have enabled to achieve high-resolution OCT imaging and extend the depth of field, especially for imaging the retina at the cellular level. Hardware-based adaptive-optics has also been successfully implemented in OCT system to compensate for aberrations directly on the experiment at the cost of an increase in the system complexity.
The aforementioned post-processing methods are phase-dependent techniques, meaning they make use of the phase information of the complex-valued tomograms; therefore, they rely on having phase-stable signals, which is a strong requirement that has hinders their implementation across all different OCT configurations.
We have developed SHARP [1], a computational adaptive-optics method that properly handles phase-stability in post-processing thus making it suitable for almost any OCT system including phase-unstable systems. We demonstrated its performance on standard, raster-scan, wavelength-swept source OCT systems without k-clocking which are strongly phase-unstable [1], illustrated in the figure below.
We further adapted SHARP for Stokes-based polarization-sensitive (PS-) OCT [2] used to retrieve the polarimetric parameters of tissue. We demonstrated the improvement of image quality provided by SHARP in the degree of polarization, and the birefringence and optic-axis orientation retrieved with the spectral-binning technique for Stokes-based PSOCT [2] as shown in the figure below.
The major drawbacks of computational methods for high-resolution OCT include i) the decrease in signal-to-noise ratio, primarily due to defocus, and ii) the challenge of achieving phase-stability in intrinsically dynamic samples such as blood vessels or cells with high intracellular activity. The latter can be overcome by imaging with ultra-fast systems, i.e., with A-lines rates in the order of MHz, or with full-field system. Alternatively, hardware-based adaptive-optics address these issues which justifies the added system complexity.
We are currently implementing an adaptive-optics polarization-sensitive OCT system for imaging living mice retina which will be a valuable tool for the study and development of treatments for eye diseases and neurodegenerative diseases that affect the central nervous system. By design, this adaptive-optics PS-OCT system will have a high 3D (axial and lateral) resolution < 2.5 µm by combining a spectral-domain detection with a broad-band light source at 850 nm and sensor-less adaptive-optics. In additional to structure and angiography imaging, this system will provide polarization-sensitive imaging functionalities.
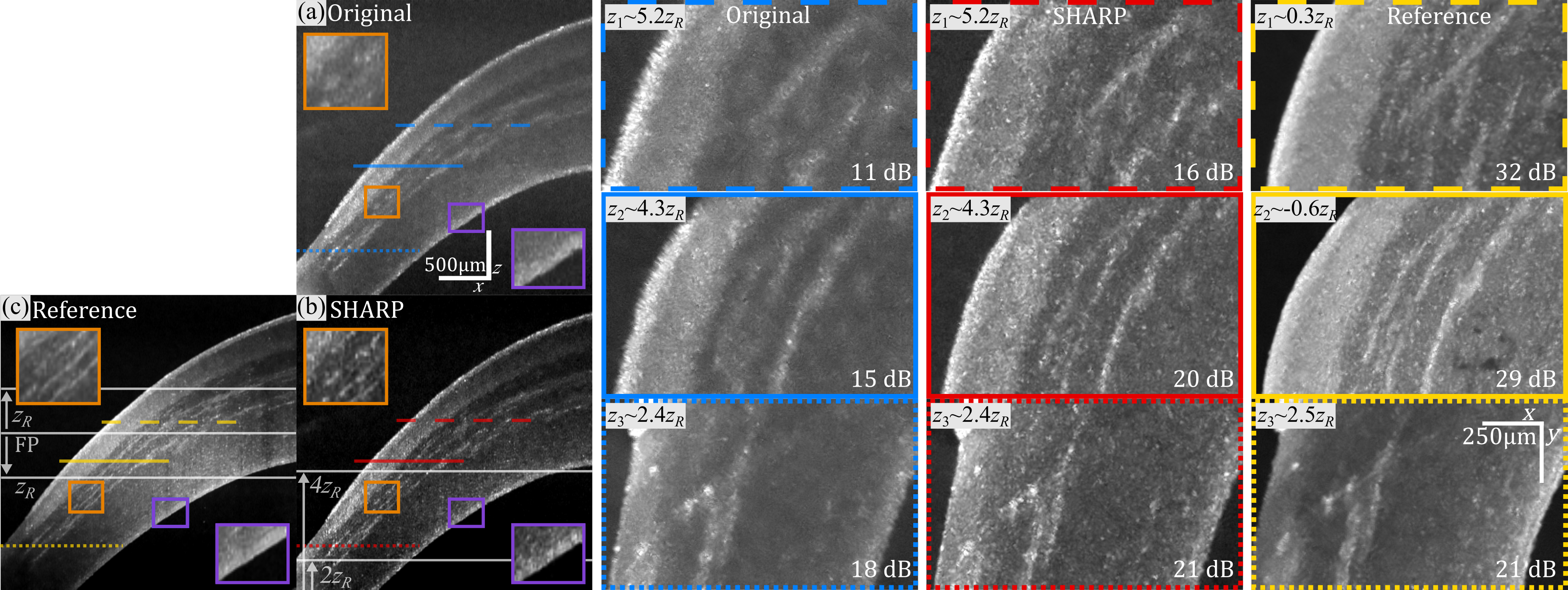
SHARP compensates for defocus in phase-unstable OCT systems. B-scan views of the anterior segment of an excised swine eye: Out-of-focus (a) before and (b) after SHARP, and (c) experimentally in-focus reference. In the original B-scan, defocus is evident in all the corneal layers and structures inside the stroma. Due to confocal gating, there is a drop in signal-to-noise ratio (SNR) due to the large offset from the focal plane. After SHARP, structures appear both sharper and with better contrast. The insets on the right show en-face views at three different distances from the focal plane (FP) indicated by the horizontal lines in the B-scan, and measured as multiple of the Rayleigh range (zR). Blurring increases toward the apex in the out-of-focus views. In contrast, SHARP views have perceptually very similar resolution at all depths, enabling improved visualization of structures inside the stroma and clearer boundaries of the corneal epithelium, resembling reference images.
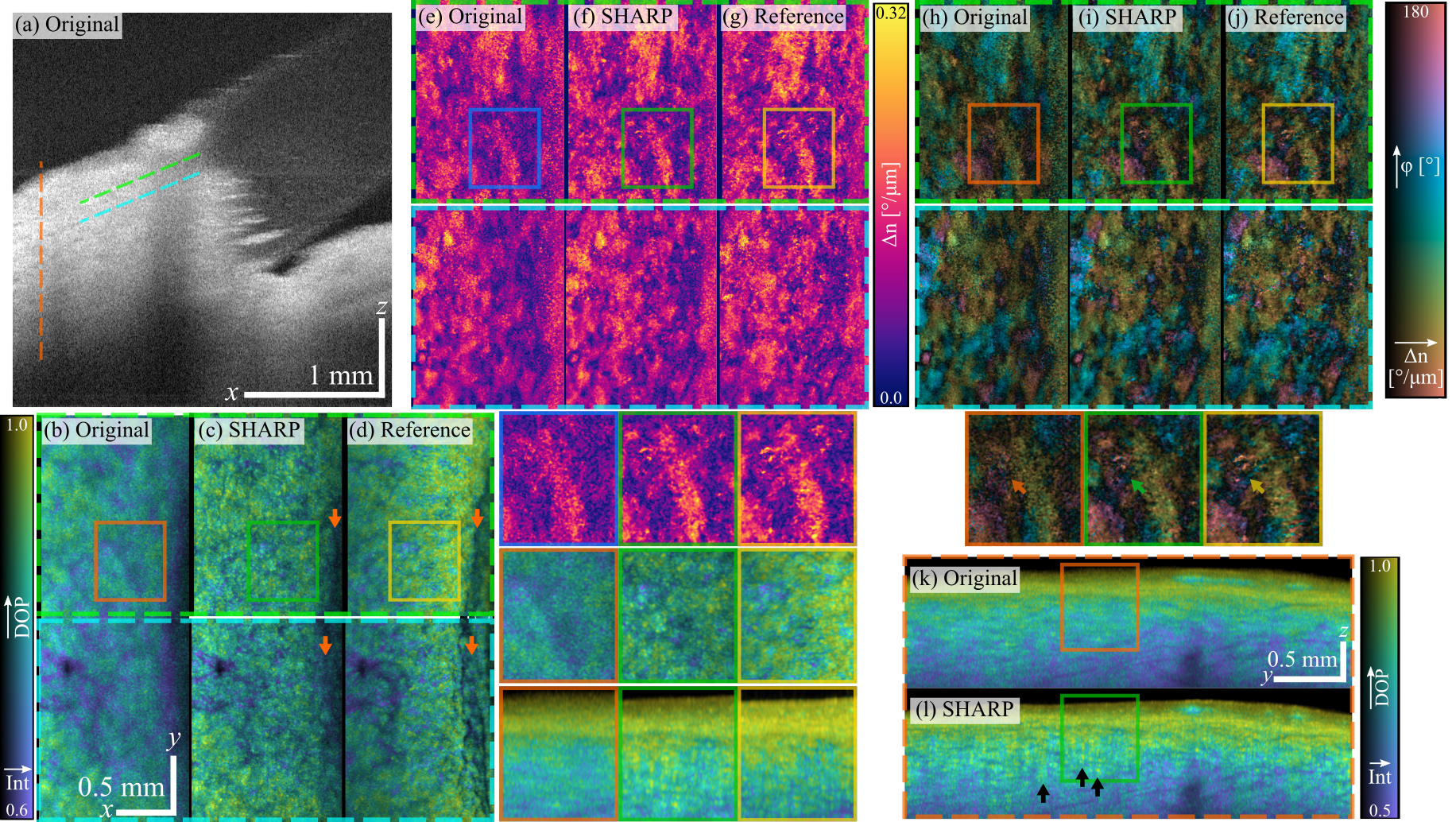
PS-SHARP compensates for defocus in polarimetric parameters of the limbal region retrieved with phase-unstable OCT systems. Imaging the limbal region in the anterior segment of the eye is challenging with medium-to-high numerical aperture given the geometry and limited depth-of-field. (a) This B-scan view is representative of medium-NA imaging of the limbus, where the axial extent of the tissue makes it impossible to have the whole ROI in focus. En-face views of tissue polarimetric parameters, showing original out-of-focus, after SHARP and reference side-to-side: (b)–(d) degree of polarization (isoluminant colormap) overlaid with intensity (brightness); (e)–(g) local phase retardation; (h)–(j) optic axis orientation (cyclic isoluminant colormap) overlaid with local phase retardation (brightness). The z–y cross section views at the location marked by the vertical line in panel (a) showing degree of polarization overlaid with intensity reveals a ridge-like structure after SHARP (l), not clearly observed in the original out-of-focus view (k).
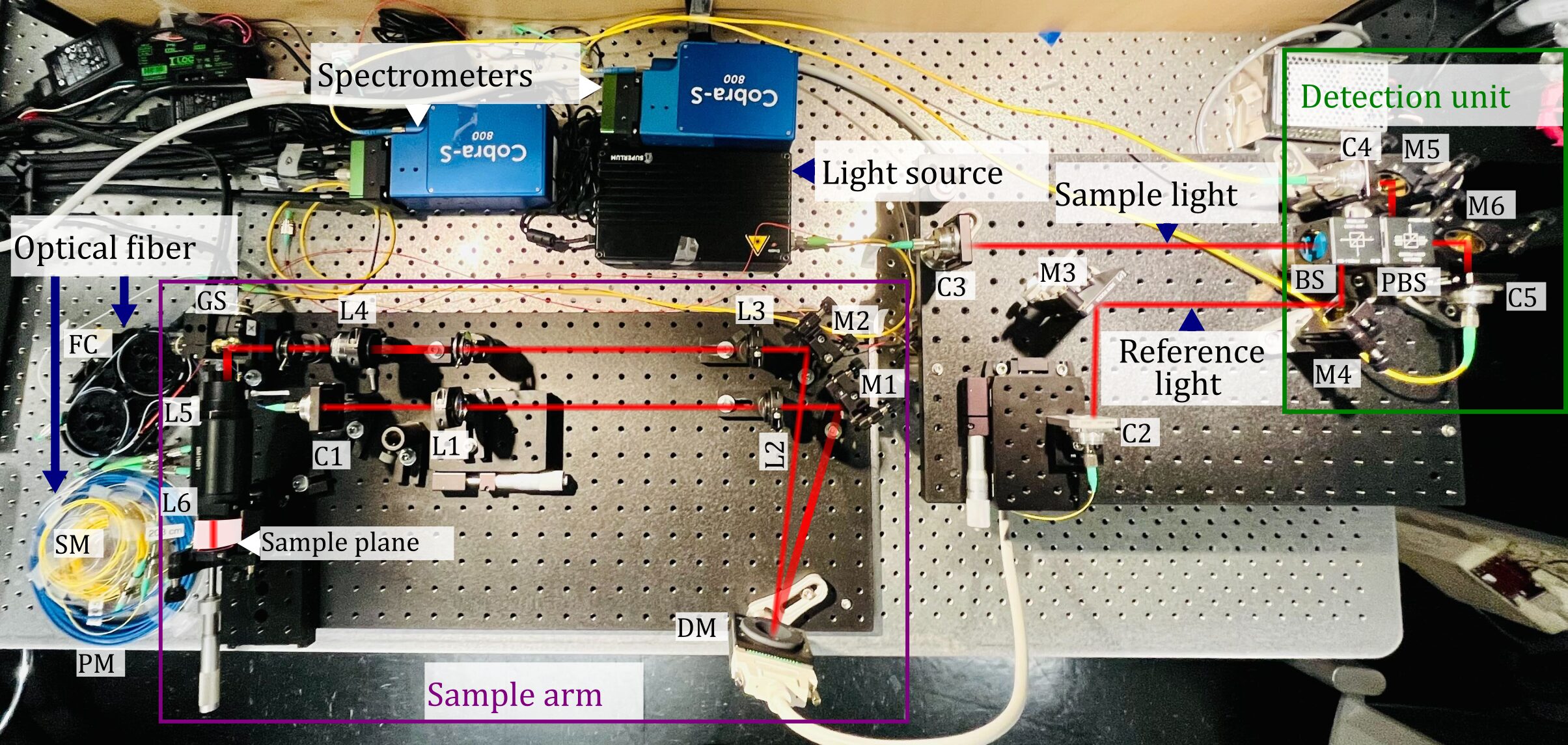
Adaptive-optics polarization-sensitive OCT system being built for high-resolution imaging of the live mouse retina. The system combines a broad-band light source, fiber-optics and free-space polarization-diverse detection coupled with two independent spectrometers for providing PS-OCT functionalities in addition to structural and angiography contrast. The sample arm integrates lenses and a deformable mirror for sensor-less adaptive-optics.
Key Researchers
Relevant Publications
- S. Ruiz-Lopera, R. Restrepo, C. Cuartas-Vélez, B. E. Bouma, and N. Uribe-Patarroyo, “Computational adaptive optics in phase-unstable optical coherence tomography.” Opt. Lett 45, 5982–5985 (2020) . (PMID: 33137049, PMCID: PMC8384075).
- S. Ruiz-Lopera, R. Restrepo, T. M. Cannon, M. Villiger, B. E. Bouma, and N. Uribe-Patarroyo, “Computational refocusing in phase-unstable polarization-sensitive optical coherence tomography.” Opt. Lett 48, 4765–4768 (2023) . (PMID: 37707897, PMCID: PMC10871002 ).

